Articles from LEAP Consulting Group

LEAP Consulting Group, a boutique digital consultancy known for guiding national clinical diagnostic laboratories through complex, multi-phase digital transformations, today announced a strategic executive appointment that underscores its expanding commitment to supporting laboratories through domain-leading expertise in commercializing high-complexity diagnostics, digitally engaging patients and providers in personalized medicine, and scaling computational biology workloads.
By LEAP Consulting Group · Via Business Wire · April 28, 2025
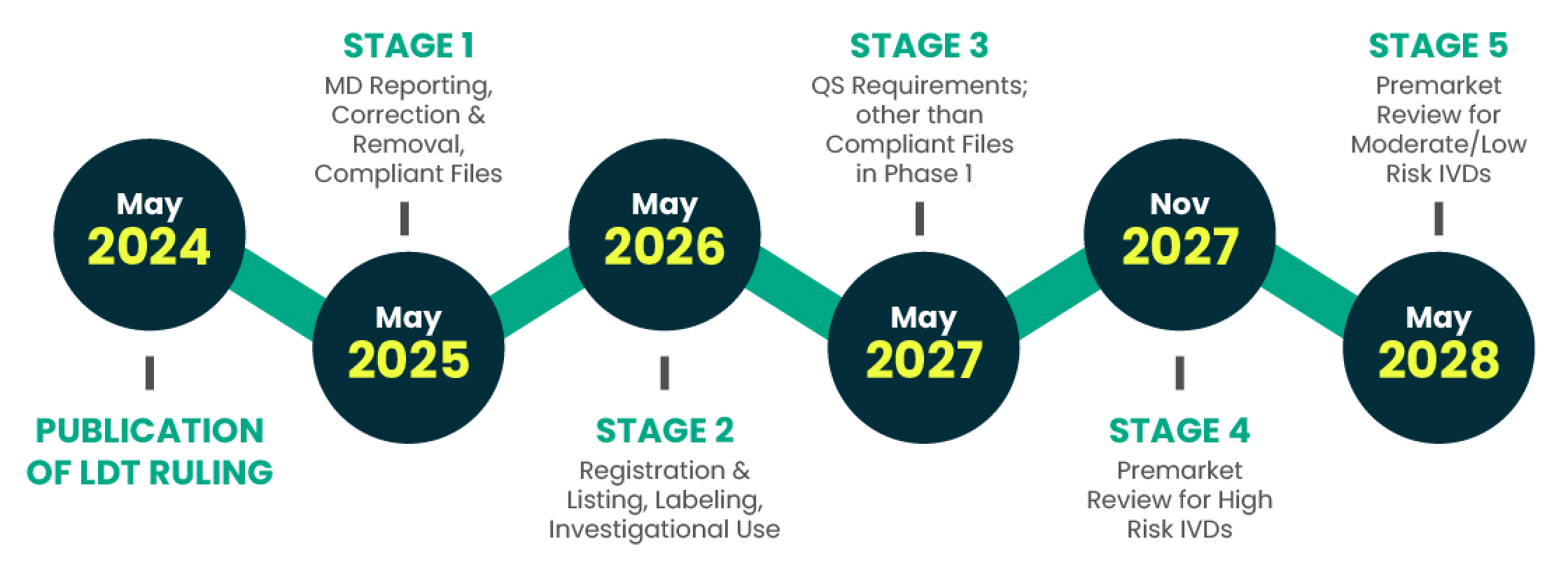
LEAP Consulting Group, a boutique digital consultancy servicing the clinical laboratory industry, is excited to announce its offering for clinical laboratories already established as CLIA certified to facilitate the planning and remediation required to extend their existing CLIA, CAP, and NYSDOH CLEP to meet the expanded requirements as recently announced by the U.S. Food and Drug Administration (FDA) as part of the FDA’s LDT Final Rule announcement declaration in May 2024. This offering specifically assists labs to understand their exposure, evaluate the best options to become compliant, inventory the specific gaps in their compliance posture, develop a level-of-effort and resource plan to comply, and support full remediation as advisors and implementation support as needed by labs. The key support LEAP is providing is to ensure clinical laboratories properly understand their exposure, budget properly to remediate in time for each phase-in deadline, and action the plan to meet the deadlines.
By LEAP Consulting Group · Via Business Wire · August 8, 2024
